Cell shape
The Cytoskeleton.
One distinguishing feature that separates the eukaryotes from prokaryotes is the presence of the cytoskeleton in the former, and apparent absence of it in the latter. This suggests that the cytoskeleton was an important factor in eukaryote evolution. The function of the cytoskeleton is to maintain cellular shape and is involved in the intracellular organization, cell polarity, cell adhesion, and in some cases, motility.
The cytoskeleton is composed of several different protein components. There are three general classes of cytoskeletal fibers: (1) microtubules, (2) intermediate filaments, and (3) actin filaments.
Microtubules
Compared to the other cytoskeletal fibers, the microtubule is rather large (15 to 35 nm diameter). Microtubules are composed of a globular protein, tubulin. The tubulin subunit is a heterodimer of alpha- and beta-tubulin. The microtubule itself is made up of 13 "proto-filaments", which are each composed of alternating alpha and beta subunits. These protofilaments are cylindrically arranged to form a hollow tube. It is the arrangement of proto-filaments that makes up the microtubule. Microtubules are polar molecules i.e. they have a fast-growing "plus end" and a slow-growing "minus end". These strands are in a constant state of flux, termed "dynamic instability" (i.e. they continuously grow and fall apart). Free GTP (guanosine triphosphate) binds to the beta-tubulin, which alters its protein structure and enables it to join to the growing end of the strand. Then, there is a delayed GTP hydrolysis reaction to yield GDP (guanosine diphosphate). Thus, at the terminus of the filament a GTP cap is present (i.e. tubulin subunits are bound to GTP), while further down the strand, tubulin subunits are bound to GDP. The presence of GDP creates a weak bond between tubulin subunits, and therefore the filament is more likely to depolymerize. In other words, if all the GTP is hydrolyzed in the filament, the filament is susceptible to falling apart (i.e. under a microscope, the microtubules are observed to shrink or even disappear). There are various proteins that are able to stabilize the microtubule, preventing de-polymerization these are known as "cap-proteins" or MAPs (Microtubule-Associated Proteins). There are also various other chemicals that can either stabilize or destabilize microtubules
Another group of cytoskeletal proteins are the intermediate filaments (IFs). IFs have an intermediate size between microtubules and actin filaments (7 to 10 nm diameter). There are, however, two general types of IFs: (1) cytoplasmic IFs, and (2) nuclear lamina. Cytoplasmic IFs are for mechanical stress and cell-to-cell junctions. Nuclear lamina create a meshwork beneath the inner nuclear membrane. One important difference to note between IF proteins and proteins for microtubules or for microfilaments, is that most IF protein subunits are filamentous, rather than globular (i.e. tubulin and actin subunits are globular).
Microfilaments
Actin filaments (or "microfilaments" these terms are used interchangeably) are the smallest of the cytoskeletal fibers (3 to 6 nm diameter). Microfilaments are flexible doubled-stranded fibers composed of polymers of the protein actin (contrast this structure to microtubules, which are hollow tubes composed of 13 protofilaments). Actin is present in all eukaryotes, and microfilaments are typically found in the cell cortex (i.e. just beneath the cell membrane). The actin subunit is globular and has a molecular mass of 43 kDa. As with microtubules, actin filaments are also dynamic and polar molecules (i.e. they have a fast growing "plus end" and a slow growing "minus end"). Free actin binds ATP (adenosine-triphosphate, as opposed to GTP in tubulin subunits) which enables polymerization, while ATP hydrolysis to ADP favours depolymerization. Unlike microtubules which undergo dynamic instability, actin filaments may use a different dynamic process.
Actin Binding Proteins.
The actin-based cytoskeleton functions for bearing of tension and for compression resistance (i.e. it is like a shock suspension system, which gives mechanical strength and maintains structural integrity of a cell). In fact, there are different microfilament arrangements for a variety of functional purposes. There are, however, two general classes of microfilament arrangement: (1) bundles (parallel and contractile), and (2) gel-like networks. Different proteins (i.e. actin-binding proteins) mediate the different arrangements. Parallel bundles are structures where microfilaments are oriented with the same polarity (i.e. plus ends are all "pointed" in the same direction) and which are closely spaced.
Flagella and Motility
Most motile move by use of flagella (flagellum) thread like locomotor appendages extending outward from the plasma membrane and cell wall. They are slender, rigid structures, about 20 nm across and up to 15 or 20 pm long. Flagella are so thin they cannot be observed directly with a bright-field microscope but must be stained with special techniques designed to increase their thickness. The detailed structure of a flagellum can only be seen in the electron microscope.
Bacterial species often differ distinctively in their patterns of flagella distribution. Monotrichous bacteria (trichous means hair) have one flagellum; if it is located at an end, it is said to be a polar flagellum. Amphitrichous bacteria (Amphi mean "on both sides") have a single flagellum at each pole. In contrast, lophotrichous bacteria (lopho means tuft) have a cluster of flagella at one or both ends. Flagella are spread fairly evenly over the whole surface of peritrichous (peri means "around") bacteria. Flagellation patterns are very useful in identifying bacteria.
Flagellar Ultrastructure : -
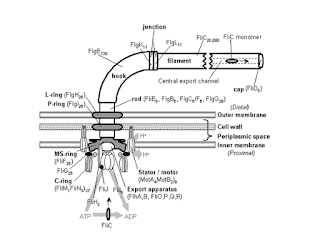
Transmission electron microscope studies have shown that the bacterial flagellum is composed of three parts. (1) The longest and most obvious portion is the filament, which extends from the cell surface to the tip. (2) A basal body is embedded in the cell; and (3) a short, curved segment, the hook, links the filament to its basal body and acts as a flexible coupling. The filament is a hollow, rigid cylinder constructed of a single protein celled flagellin, which ranges in molecular weight from 30,000 to 60,000. The filament ends with a capping protein. Some bacteria have sheaths surrounding their flagella. For example Vibrio cholerae has a lipopolysaccharide sheath.
The hook and basal body are quite different from the filament of different protein subunits. The basal body is the most complex part of a flagellum. In E.coli and most gram-negative bacteria, the body has four rings connected to a central rod. The outer L and P rings associate with the lipopolysaccharide and peptidoglycan layers, respectively. The inner M ring contacts the plasma membrane. Gram-positive bacteria have only two basal body rings, an inner ring connected to the plasma membrane and an outer one probably attached to the peptidoglycan.
Flagellar Synthesis
The synthesis of flagella is a complex process involving at least 20 to 30 genes. Besides the gene for flagellin, 10 or more genes code for hook and basal body proteins; other genes are concerned with the control of flagellar construction or function. It is not known how the cell regulates or determines the exact location of flagella.
The Mechanism of Flagellar Movement
Procaryotic flagella operate differently from eukaryotic flagella. The filament is in the shape of a rigid helix, and the bacterium moves when this helix rotates. Considerable evidence shows that flagella act just like propellers on a boat. Bacterial mutants with straight flagella or abnormally long hook regions (polyhook mutants) cannot swim. When bacteria are tethered to a glass slide using antibodies to filament or hook proteins, the cell body rotates rapidly about the stationary flagellum. If polystyrene-latex beads are attached to flagella, the beads spin about the flagellar axis due to the flagellar rotation. The flagellar motor can rotate very rapidly. The E. coli motor rotates 270 revolutions per second; Vibrio alginolyticus averages 1,100 rps.
The direction of flagellar rotation determines the nature of the bacterial movement. Monotrichous, polar flagella rotate slowly clockwise. The rotating helical flagellar filament thrusts the cell forward in a run with the flagellum trailing behind. Monotrichous bacteria stop and tumble randomly by reversing the direction of flagellar rotation. Peritrichously flagellated bacteria operate in a somewhat similar way to move forward, the flagella rotate counterclockwise. As they do so, they bend at their hooks to form a rotating bundle that propels them forward. Clockwise rotation of the flagella disrupts the bundle and the cell tumbles.
Because bacteria swim through rotation of their rigid flagella, there must be some sort of motor at the base. A rod or shaft extends from the hook and ends in the M ring, which can rotate freely in the plasma membrane. It is believed that the S ring is attached to the cell wall in gram-positive cells and does not rotate. The P and L rings of gram-negative bacteria would act as bearings for the rotating rod. There is some evidence that the basal body is a passive structure and rotates within a membrane-embedded protein. The relationship of flagellar rotation to bacterial movement. The rotor is like an electrical motor turns in the center of a ring of electromagnets (the stator).
The exact mechanism that drives basal drives basal body rotation still is not clear provides a more detailed depiction of the basal body in gram-negative bacteria. The rotor portion of the motor seems to be made primarily of a rod, the M ring, and C ring joined to it on the cytoplasmic side of the basal body. These two rings are made of several proteins. The two most important proteins in the stator part of the motor are Mot A and Mot B. These form a proton channel through the plasma membrane and Mot B also anchors the Mot complex to cell wall peptidoglycan. There is some evidence that Mot A and G directly interact during flagellar rotation. This rotation is driven by proton or sodium gradients in prokaryotes, not directly by ATP as is the case with eukaryotic flagella.
Bacteria can move by mechanisms other than flagellar rotations. Spirochetes are helical bacteria that travel through viscous substances such as mucus or mud by flexing and spinning movements caused by a special axial filament composed of periplasmic flagella. A very different type of motility, gliding motility, is employed by many bacteria: cyanobacteria.



Comments
Post a Comment